Explain the Difference Between an Ion and an Isotope
Are all atoms of an element the same. The technique is often used in reversed-phase chromatography as a convenient method to control the retention of solutes.
Isotopes Vs Ions What Is The Difference Youtube
The ε Hf t values are normalised to a 01 difference between the sample and the chondritic reservoir at the time of magma crystallisation.
. State the difference if any in the chemistry of these isotopes. Our simulations predict a high κ for crystals of all boron isotope compositions. We measured the isotope compositions of the cBN crystals using time-of-flight secondary ion mass spectrometry TOF-SIMS.
16 26 21 18. Could potentially explain the difference between experiment and theory Fig. For example while boron forms only BF4 aluminium gives AlF 6 3 ion.
Explain why it would be difficult to distinguish between 48Ti2 and 24Mg ions using a mass spectrometer. There is typically not enough energy to fragment the molecular ions thus increasing the strength of signal produced by these ions. The combined effect of.
The difference between these reactions is easy to understand. Some metals can satisfy a portion of their combining power by binding. To promote equity and diversity among authors reviewers and editors.
Reuters the news and media division of Thomson Reuters is the worlds largest multimedia news provider reaching billions of people worldwide every day. How can you tell one isotope from another. The principle may for example be applied to direct chiral separations using either.
What is the atomic mass of this isotope. The Na ion formed has only two shells which are complete Na. The magnitudes of such secondary isotope effects at the α-carbon atom are largely determined by the C α-HD vibrationsFor an S N 1 reaction since the carbon atom is converted into an sp 2 hybridized carbenium ion during the transition state for the rate-determining step with an increase in C α-HD bond order an inverse kinetic isotope effect would be expected if only the.
Between the 3p and the 4s levels of energy. The two species at each x coordinate represent the two cations present in the multisalt feed solution. 3A gray bars along with the variation across multiple samples and measurements.
K2 L8 Question 10. The presence of these d-orbitals influences the chemistry of the heavier elements in a number of other ways. The basic principle is that mixtures of two components that have different isotope ratios eg 87 Sr 86 Sr or 15 N 14 N and different concentrations of the element in question eg Sr or N form hyperbolas when plotted on diagrams with coordinates of isotope ratios versus concentration.
Use the sim to learn about isotopes and how abundance relates to the average atomic mass of an element. Build an Atom - PhET Interactive Simulations. Falkenherz to add to what MA ROger has already said if you want a specific mechanism the transport of carbon dioxide between the atmosphere and surface oceans is proportional to the difference in partial pressure of CO2 between air and ocean.
Using these d-orbitals the third period elements can expand their covalence above four. The decay constant for 176 Lu is 1865 10 11 yr 1 and the chondritic ratios are 0282772 for 176 Hf 177 Hf and 00332 for 176 Lu 177 Hf Blichert-Toft and Albarède 1997 Scherer et al 2001. The IDA binding energy difference was calculated as the binding energy of the strong binding species Cu 2 minus the binding energy of the weaker binding species Ni 2 Zn 2 Co 2 or Mg.
The same number of atoms and different masses. The mission of Urology the Gold Journal is to provide practical timely and relevant clinical and scientific information to physicians and researchers practicing the art of urology worldwide. The first reaction uses a nonpolar reagent to reduce a nonpolar double bond.
In ion pair chromatography the solute ion is distributed between the mobile and the stationary phase together with an ion of opposite charge a so-called counterion. As the difference between the elemental concentrations of two components endmembers. Na ion is formed when Na atom loses one electron from the valence shell ie.
C _____ are groups of the same kinds of atoms that cannot be broken down into other substances by ordinary chemical means. Both compounds are nonpolar hydrocarbons. The spatial resolutions of even the most sensitive isotope analysis techniques based on light or ion probes are limited to a few hundred nanometres.
To provide a platform for discussion of current ideas in urologic education patient engagement. An isotope of Oxygen has 8 protons 10 neutrons and 8 electrons. Of an inert external salt with a common counterion 2BN to soln.
Discriminating between two outer-sphere ion pairs having a naked β-agostic cation. Compare 1 mole of hydrogen with 1 mole of oxygen. Adduct ion MH where a proton H is added to the molecule.
Which of the following forces is primarily responsible for this difference. Calculate the number of. Therefore if we increase the partial pressure of CO2 in the atmosphere eg.
The main difference between these ionization techniques is in the amount of energy that is transferred to the molecule and the type of ions generated. C Relationship between ion binding energy to IDA and normalized ion flux. Electrons orbit around the nucleus in defined paths like planets orbit the Sun.
Although vibrational spectroscopy using. The atoms on the surface of a metal are different from those buried in the body of the solid because they cannot satisfy their tendency to form strong metal-metal bonds. By burning fossil fuels then this.
The relative reactivity difference between ion pairs and larger ionic aggregates is similar in both solvents about 4 and 3 increment on passing from N 10 to N 16 in methylcyclohexane and toluene resp. 10 b State any differences and similarities in the atomic structure of the isotopes of an element. K2 L8 M1.
A mole is defined as the number of atoms in an exact mass of which isotope. If the element bromine is in the form two isotopes which are 79 35 Br 497 and 81 35 Br 303 then calculate the average atomic. Information you can trust.
What Are The Differences Between Ions And Isotopes Quora
Atoms Isotopes And Ions Atoms I Parts Nucleus Protons Neutrons Orbiting Nucleus Electrons Ppt Download
Isotopes And Ions Teaching Resources

Difference Between Atoms And Ions Explanation Examples Youtube
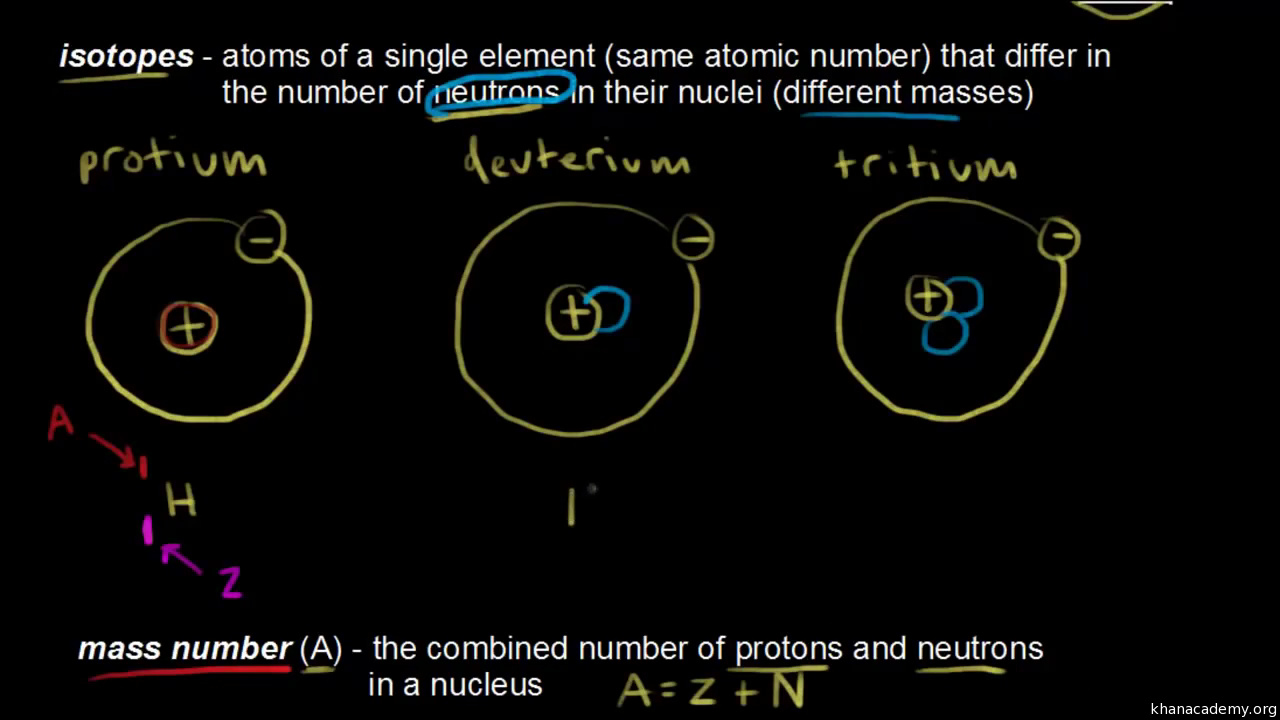
Atomic Number Mass Number And Isotopes Video Khan Academy

Atomic Structure Isotopes Ions Quiz Proprofs Quiz

4 3 Notes Elements Isotopes Ions Ppt Video Online Download
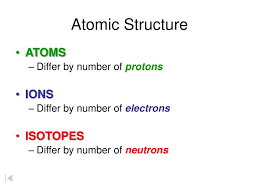
Atoms Ions And Isotopes Atoms Molecules Quiz Quizizz

Difference Between Atoms Ions And Isotopes Teaching Resources

Atoms Ions And Isotopes Ppt Video Online Download

Isotopes Vs Ions The Difference Between Isotopes And Ions Youtube
What Are The Differences Between Ions And Isotopes Quora
Atoms Isotopes Ions And Molecules Boundless Biology
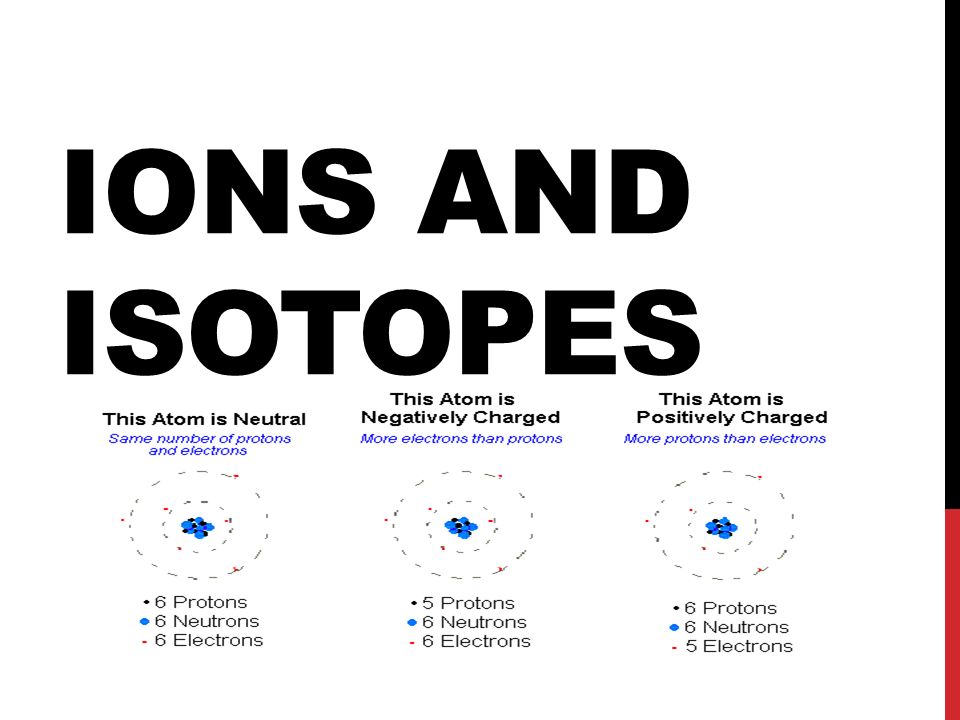
Ions And Isotopes Characteristics Of Ions Ions Are Particles With A Charge All Ions Begin As Neutral Atoms Atoms That Have Lost Electrons Are Called Ppt Download

Atoms Ions And Isotopes Ppt Video Online Download

Difference Between Ions And Isotopes With Table Ask Any Difference

Worked Example Identifying Isotopes And Ions Video Khan Academy

Ions And Isotopes Objective Students Will Be Able To Ppt Video Online Download
